Doxycyclin Hyclat
Hannergrond
Doxycycline Hyclate ass en Antibiotikum [1].
Doxycycline Hyclate ass en Derivat vun Tetracyclin a besëtzt d'Aktivitéite vun anti-inflammatoreschen an antimikrobiellen. Doxycyclin hemmt Dengue Virus Replikatioun in vitro mat enger Temperaturofhängeger Manéier. Den IC50 Wäert ass 52.3μM bei 37°C an 26.7μM bei 40°C. Et hemmt den Dengue Virus duerch d'Inhibitioun vun der NS2B-NS3 Serinprotease vum Virus. 60μM Doxycyclin reduzéiert den CPE vun den DNEV2-infizéierte Zellen [1].
Doxycyclin gëtt als Inhibitor vu MMP fonnt. Doxycycline Behandlung reduzéiert MMP-8 an -9 Niveauen an hemmt den Ausdrock vun Tissue MMP-2 an MMP-9. Ausserdeem reduzéiert d'Behandlung mat Doxycyclin d'Heefegkeet vun intrakranialen Aneurysmen wesentlech. Doxycyclin ass och gemellt ginn als en anti-inflammatoreschen Agent baséiert op senger Inhibitioun vu Matrixmetalloproteinasen. Zousätzlech, huet doxycycline mächteg antimalarial Aktivitéit mat IC50 Wäert vun 320nM bei 96h kënschtlech [2, 3].
Referenzen:
[1] Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Arch Virol. Abrëll 2014;159(4):711-8.
[2] Maradni A, Khoshnevisan A, Mousavi SH, Emamirazavi SH, Noruzijavidan A. Roll vun Matrixmetalloproteinasen (MMPs) an MMP-Inhibitoren op intrakranialen Aneurysmen: e Bewäertungsartikel. Med J Islam Republik Iran. 2013 Nov;27(4):249-254.
[3] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, et al. Nelson ML. In vitro an in vivo antimalarial Effizienz vun optiméierten Tetracyclinen. Antimicrob Agenten Chemother. 2013 Jul;57(7):3131-6.
Beschreiwung
Doxycycline (Hyclate) (Doxycycline Hydrochlorid Hemiethanolate Hemihydrat), en Antibiotikum, ass en oral aktiven a breetspektrum Metalloproteinase (MMP) Inhibitor [1].
Klinesch Prozess
| NCT Zuel | Sponsor | Zoustand | Start Datum | Phase |
| NCT00246324 | Louisiana State University Health Sciences Center Shreveport|Biogen | Multiple Sklerose | Dezember 2003 | Phase 4 |
| NCT00910715 | Universitéit Medical Center Ljubljana | Erythema Chronicum Migrans | Juni 2009 | Net applicabel |
| NCT00243893 | Universitéit vu Kalifornien, San Francisco | National Institut fir Neurologesch Stéierungen a Schlaganfall (NINDS) | Aneurysmen|Arteriovenöse Malformatiounen | Juli 2004 | Phase 1 |
| NCT00126399 | CollaGenex Pharmaceuticals | Rosacea | Juni 2004 | Phase 3 |
| NCT01318356 | Radboud University|ZonMw: The Netherlands Organization for Health Research and Development | Q Féiwer | Middegkeet Syndrom, Chronesch | Coxiella Infektioun | Abrëll 2011 | Phase 4 |
| NCT00177333 | Universitéit vu Pittsburgh | Ofdreiwung, Induzéiert|Erbriechen | September 2005 | Phase 4 |
| NCT00007735 | US Department of Veterans Affairs|Pfizer|United States Department of Defense|VA Office of Research and Development | Persesche Golf Syndrom|Mycoplasma Infektiounen | Januar 1999 | Phase 3 |
| NCT00351273 | Universitéit vu South Florida | National Institut fir Arthritis a Muskuloskeletal a Hautkrankheeten (NIAMS) | Arthritis, Reaktiv|Reiter Krankheet | Mee 2006 | Phase 3 |
| NCT00469261 | Careggi Spidol | Myokardial Infarkt|Lénks Ventrikulär Remodeling | Mee 2007 | Phase 2 |
| NCT00547170 | Universitéit vu Pittsburgh | Tu Du Spidol | Endometritis | Januar 2007 | Phase 4 |
| NCT01475708 | Universitéit Medical Center Ljubljana | Lyme Borreliosis | Mee 2011 |
|
| NCT01368341 | Morten Lindbaek|Norwegian Institute of Public Health|Sorlandet Hospital HF|Norwegian University of Life Sciences|University of Oslo | Erythema Migrans|Erythema Chronicum Migrans|Borreliosis|Lyme Krankheet|Fréi Lyme Krankheet | Juni 2011 | Phase 4 |
| NCT02538224 | Islamesch Azad Universitéit, Teheran | Chronesch Parodontitis | Juli 2013 | Phase 2|Phase 3 |
| NCT00066027 | Universitéit Nebraska | National Institut fir Dental a Craniofacial Fuerschung (NIDCR) | Parodontitis | Juni 2002 | Phase 3 |
| NCT00376493 | Hospital de Clinicas de Porto Alegre | Septesch Ofdreiwung | Mee 2006 | Phase 4 |
| NCT03448731 | Fundacion CRIS de Investigación fir Vencer el Cáncer|Amgen|Apices Soluciones SL | Hauttoxizitéit | Mee 10, 2018 | Phase 2 |
| NCT00989742 | Universitéit Nottingham | Lymphangioleiomyomatosis | Tuberous Sklerose | Juli 2009 | Phase 4 |
| NCT01438515 | Horizon Health Network | Methicillin-resistente Staphylococcus Aureus | August 2008 | Net applicabel |
| NCT02929121 | D'Task Force fir Global Gesondheet | United States Agency for International Development (USAID) | Lymphödem | Lymphatesch Filariasis | Filariasis | Januar 15, 2019 | Phase 3 |
| NCT00952861 | Odense University Hospital|Kolding Sygehus|Svendborg Hospital|Fredericia Hospital|Naestved Hospital|Hillerod Hospital, Dänemark|Region Syddanmark|Danmarks Lungeforening|Danish National Research Foundation | Pulmonaler Krankheet, chronesch Obstruktiv | Oktober 2009 | Phase 4 |
| NCT00138801 | Sorlandet Spidol HF | Lyme Neuroborreliosis | März 2004 | Phase 3 |
| NCT00942006 | Universitéit Medical Center Ljubljana | Verdächtegt fréi Lyme Neuroborreliose | Juli 2009 | Net applicabel |
| NCT02713607 | Universitéit vu Kalifornien, Davis | Akne Vulgaris | Mäerz 2016 | Phase 1|Phase 2 |
| NCT00560703 | Galderma | Blepharitis|Meibomianitis|Dréchent Auge | November 2007 | Phase 2 |
| NCT01014260 | Johns Hopkins Universitéit | Kardiovaskulär Krankheet | September 2010 | Phase 4 |
| NCT00000938 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Lyme Krankheet | Phase 3 | |
| NCT01398072 | University College, London|Royal Free Hampstead NHS Trust|University of Cambridge|National Institute for Health Research, Vereenegt Kinnekräich | Chronic Obstructive Pulmonary Disease (COPD). | Dezember 2011 | Phase 3 |
| NCT03479502 | Vanderbilt University Medical Center|Orthopedic Research and Education Foundation | Adhesive Capsulitis|Adhesive Capsulitis vun Onspezifizéierter Schëller|Frozen Shoulder | Januar 5, 2018 | Phase 4 |
| NCT02929134 | D'Task Force fir Global Gesondheet | United States Agency for International Development (USAID) | Lymphödem | Lymphatesch Filariasis | Filariasis | 16. Februar 2018 | Phase 3 |
| NCT00480532 | Oregon Health and Science University | Kontrazeptiva, Oral | Mee 2007 | Net applicabel |
| NCT01594827 | Johns Hopkins University|Case Western Reserve University|Cystic Fibrosis Foundation | Cystesch Fibrose | Oktober 2012 | Phase 2 |
| NCT01744093 | Weill Medical College vun der Cornell University | National Heart, Lung, and Blood Institute (NHLBI) | HIV|Chronesch Obstruktiv Pulmonalerkrankheet (COPD)|Emphysem | 17. Juli 2014 | Net applicabel |
| NCT03530319 | National Taiwan Universitéit Spidol | Pneumonie, Mycoplasma | November 10, 2018 | Net applicabel |
| NCT04167085 | Universitéit vu Kalifornien, Los Angeles | Epistaxis | 18. Dezember 2017 | Phase 4 |
| NCT01411202 | Ottawa Spidol Fuerschung Institut | Malignant Pleural Effusioun | Juni 2011 | Phase 2 |
| NCT01474590 | Galderma | Akne | November 2011 | Phase 3 |
| NCT00649571 | Mylan Pharmaceuticals | Gesond | Juli 2005 | Phase 1 |
| NCT02899000 | Galderma Laboratories, LP | Akne Vulgaris | 29. Juli 2016 | Phase 4 |
| NCT00538967 | Leiden University Medical Center | Aorta Aneurysmus, Bauch | Mee 2002 | Phase 2 |
| NCT00439400 | D'Geschicht vun Alacrity Biosciences, Inc. | Dréchen Aen | Februar 2007 | Phase 2 |
| NCT00917553 | Thomas Gardner|Penn State University|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetesch Retinopathie | Juli 2009 | Phase 2 |
| NCT00495313 | CollaGenex Pharmaceuticals | Rosacea | März 2007 | Phase 4 |
| NCT01855360 | Brigham a Fraen Spidol | Amyloidose; Häerz (Manifestatioun)|Senile Häerzamyloidose | Juni 2013 | Phase 1|Phase 2 |
| NCT00419848 | Shahid Beheshti Universitéit vu Medizinesche Wëssenschaften | Akne | August 2006 | Phase 2 |
| NCT03532464 | University Hospital, Bordeaux|USC EA 3671 Infections humaines à mycoplasmes et à chlamydiae | Chlamydia Trachomatis Infektioun|Vaginal Infektioun|Anal Infektioun | 1. Juli 2018 | Phase 4 |
| NCT02756403 | Medstar Health Research Institute|Society of Family Planning | Éischt Trimester Ofdreiwung | Mäerz 2016 | Net applicabel |
| NCT00353158 | National Institut fir Arthritis a Muskuloskeletal a Hautkrankheeten (NIAMS) | National Instituter fir Gesondheet Clinical Center (CC) | Gesond Fräiwëlleger|Pilz Infektiounen|Bakteriell Infektiounen | 11. Juli 2006 | Phase 1 |
| NCT01317433 | Institut Cancerologie de l'Ouest | Colorectal Kriibs Metastatesch|Hauttoxizitéit | Dezember 2010 | Phase 3 |
| NCT01658995 | Petra M. Casey|Mayo Klinik | ESI-verbonne Blutungen | 13. September 2012 | Phase 3 |
| NCT03968562 | State University of New York - Downstate Medical Center | Hives | Mee 15, 2019 | Phase 2 |
| NCT02569437 | Icahn School of Medicine um Mount Sinai | Polyp vum Nasal Sinus | September 2014 | Phase 2 |
| NCT01198509 | NYU Langone Health|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|Memorial Sloan Kettering Cancer Center | Rheumatoid Arthritis|Psoriatesch Arthritis|Periodontal Krankheet | Januar 2010 | Net applicabel |
| NCT01163994 | Universitéit Medical Center Ljubljana | Multiple Erythema Migrans | Juni 2010 | Net applicabel |
| NCT02388477 | Milton S. Hershey Medical Center | Rotator Cuff Verletzung | Net applicabel | |
| NCT01010295 | International Extranodal Lymphoma Study Group (IELSG) | Non-Hodgkin Lymphom | September 2006 | Phase 2 |
| NCT00775918 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Gesond | Juni 2005 | Net applicabel |
| NCT04050540 | Universitéit vu Washington|Kenya Medical Research Institute|Kenya National AIDS & STI Kontrollprogramm|University of California, San Francisco|National Institute of Allergy and Infectious Diseases (NIAID) | HIV Infektiounen|HIV+AIDS|Neisseria Gonorrheae Infektioun|Chlamydia Trachomatis Infektioun|Syphilis Infektioun | 5. Februar 2020 | Phase 4 |
| NCT02562651 | Russesch Akademie vun Medical Sciences | Kardiovaskulär Krankheeten | Akute Myokardinfarkt | Februar 2014 | Phase 2|Phase 3 |
| NCT00001101 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Lyme Krankheet | Phase 3 | |
| NCT00340691 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | National Instituter fir Gesondheet Clinical Center (CC) | Mansonella Perstans Infektioun|Mp Mikrofilarämie | 6. Dezember 2004 | Phase 2 |
| NCT01112059 | Universitéit vun Alabama zu Birmingham | Cystic Fibrosis Foundation | Cystesch Fibrose | November 2008 | Net applicabel |
| NCT00652704 | Par Pharmaceutical, Inc.|Anapharm | Fir d'Bioequivalenz ënner Fed Konditiounen ze bestëmmen | Juli 1999 | Phase 1 |
| NCT01783860 | Teheran University of Medical Sciences | Posterior Blepharitis | Januar 2013 | Phase 2 |
| NCT02564471 | State University of New York - Upstate Medical University|Walter Reed Army Institute of Research (WRAIR)|Kansas State University | Tollwut | Abrëll 2016 | Phase 4 |
| NCT04206631 | Indonesien Universitéit | Akne Vulgaris | Abrëll 1, 2015 | Phase 1 |
| NCT03956446 | University Medical Center Ljubljana|University of Ljubljana School of Medicine, Slowenien | Tick Borne Encephalitis | September 1, 2014 | Net applicabel |
| NCT03960411 | Felix Chikita Fredy, MD|National Cardiovascular Center Harapan Kita Spidol Indonesien|Indonesia University | ST Héicht Myokardinfarkt|Anterior Wall Myokardialinfarkt|Häerzfehler|Remodeling, Ventrikulär | Mee 25, 2019 | Phase 3 |
| NCT00322465 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Urethritis | November 2006 | Phase 2 |
| NCT01375491 | Universitéit vu Kalifornien, San Diego|Ruth L. Kirschstein National Research Service Award|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)|National Center for Research Resources (NCRR) | Typ 2 Diabetis | Adipositas | Oktober 2009 | Phase 4 |
| NCT03478436 | Medical University of Vienna|Dr. Reddy's Laboratories Limited Kapitaléierung | Rosacea | Juli 2016 | Phase 1 |
| NCT01207739 | Radboud University|Sint Maartenskliniek|ZonMw: The Netherlands Organization for Health Research and Development | Lyme Krankheet | Borrelia Infektioun | September 2010 | Phase 4 |
| NCT00939562 | Pfizer | Bakteriell Infektioun | November 2008 | Phase 4 |
| NCT03608774 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Anal Chlamydien Infektioun | 26. Juni 2018 | Phase 4 |
| NCT02281643 | Kwame Nkrumah University of Science and Technology|University of Bonn|Heinrich-Heine University, Düsseldorf | Mansonella Perstans Infektioun|Buruli Ulcer|Tuberkulose|Co-Infektioun | Oktober 2014 | Phase 2 |
| NCT00066066 | Forsyth Institut National Institut fir Dental a Craniofacial Fuerschung (NIDCR) | Periodontitis|Periodontal Krankheeten | Juli 2003 | Phase 2 |
| NCT01798225 | Medical University of South Carolina|National Center for Research Resources (NCRR) | Periodontal Krankheet | Typ 2 Diabetis mellitus | Dezember 2007 | Phase 4 |
| NCT00612573 | Warner Chilcott | Akne Vulgaris | Februar 2008 | Phase 2 |
| NCT01631617 | National Institut fir Arthritis a Muskuloskeletal a Hautkrankheeten (NIAMS) | National Instituter fir Gesondheet Clinical Center (CC) | Ekzeme|Dermatitis|Hautkrankheeten, Genetesch|Dermatitis, Atopesch|Hautkrankheeten | 18. September 2012 | Phase 2 |
| NCT03173053 | Radboud University|ZonMw: The Netherlands Organization for Health Research and Development|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Aalborg University Hospital|Rigshospitalet, Dänemark | Staphylococcus Aureus|Motilitéitsstéierung | 8. Februar 2018 | Net applicabel |
| NCT00715858 | McMaster University|The Physicians' Services Incorporated Foundation | Alzheimer Krankheet | Mee 2008 | Phase 3 |
| NCT03584919 | Universitéit Medical Center Ljubljana | Erythema Chronicum Migrans | 1. Juni 2006 | Net applicabel |
| NCT01469585 | Universitéit vun Hawaii|Charles Drew Universitéit fir Medizin a Wëssenschaft|Meharry Medical College | Duerchbroch Blutungen | November 2011 | Net applicabel |
| NCT02759120 | Weill Medical College vun der Cornell University|Duke Clinical Research Institute|University of Chicago|University of Washington|University of Pittsburgh|National Heart, Lung, and Blood Institute (NHLBI) | Idiopathesch Pulmonal Fibrose | 22. Mäerz 2017 | Phase 3 |
| NCT02735837 | Amirhossein Farahmand|Islamesch Azad Universitéit, Teheran | Diabetis mellitus mat Periodontal Krankheet | Januar 2015 | Phase 2|Phase 3 |
| NCT03655197 | Universitéit vu Kalifornien, Davis | Rosacea|Okulär Rosacea|Haut Rosacea | 2. November 2017 | Fréier Phase 1 |
| NCT01188954 | Northwell Gesondheet | Seroma | Januar 2010 | Net applicabel |
| NCT00388778 | Shahid Beheshti Universitéit vu Medizinesche Wëssenschaften | Akne|Entzündung | Oktober 2005 | Phase 2|Phase 3 |
| NCT01087476 | Metropolitan Autonomous University|Instituto Nacional de Cancerologia de Mexico | Mucositis | Mee 2010 | Phase 2 |
| NCT02174757 | CD Pharma Indien Pvt. Ltd.|Sree Mookambika Institut fir Dental Sciences | Chronesch Parodontitis | August 2014 | Phase 3 |
| NCT03911440 | National Taiwan Universitéit Spidol | Atypesch Pneumonie | November 10, 2018 | Net applicabel |
| NCT02553083 | Rabin Medical Center | Bakteriell Infektioun duerch Helicobacter Pylori (H. Pylori) | 22. Oktober 2015 | Phase 4 |
| NCT04234945 | Ahmadu Bello Universitéit Teaching Spidol | Onfruchtbarkeet, weiblech | Pelvic inflammatoresch Krankheet | Januar 13, 2020 | Net applicabel |
| NCT00892281 | Galderma Laboratories, LP | Rosacea | Abrëll 2009 | Phase 4 |
| NCT02913118 | Qingfeng Pharmaceutical Group | Gemeinschaft Acquired Pneumonie | Juli 2016 | Phase 4 |
| NCT04153604 | Methodist Gesondheetssystem | Zirrhose | Spontan bakteriell Peritonitis | November 4, 2019 |
|
| NCT03153267 | University Medical Center Ljubljana|University of Ljubljana School of Medicine, Slowenien | Erythema Chronicum Migrans | 1. Juni 2017 | Net applicabel |
| NCT03116659 | James J. Peters Veteranen Affären Medical Center | Lymphom, T-Zell, Haut | 1. Februar 2018 | Fréier Phase 1 |
| NCT03401372 | Jian Li|Peking University First Hospital|Chinese PLA General Hospital|Beijing Chao Yang Hospital|West China Hospital Affiliated with Sichuan University|Tongji Hospital Affiliated with Tongji Medical College of HUST|Union Hospital Affiliated with Tongji Medical College of HUST|Shanghai Changzheng Hospital| Nanfang Spidol vun der Southern Medical University|Peking Union Medical College Hospital | Amyloidose; Systemesch | Abrëll 21, 2018 | Net applicabel |
| NCT01380496 | Par Pharmaceutical, Inc.|Anapharm | Fir d'Bioequivalenz ënner Fed Konditiounen ze bestëmmen | November 1999 | Phase 1 |
| NCT03083197 | Universitéit Oxford|Shoklo Malaria Fuerschung Eenheet|Chiangrai Prachanukroh Spidol | Scrub Typhus | 15. Oktober 2017 | Phase 4 |
| NCT00237016 | Medical Corps, Israel Defense Force | Relapsing Fever, Tick-Borne|Jarisch Herxheimer Reaktioun | Abrëll 2002 | Phase 2|Phase 3 |
| NCT01308619 | Galderma Laboratories, LP | Rosacea | Abrëll 2011 | Phase 4 |
| NCT01198912 | University Hospital, Gent | Chronesch Rhinosinusitis | Nasal Polypen | 22. November 2011 | Phase 2 |
| NCT02016365 | Umeå Universitéit | Transthyretin Amyloidose|Kardiomyopathie | Februar 2012 | Phase 2 |
| NCT00783523 | Universitéit vu Kalifornien, San Francisco | Arteriovenöse Malformatiounen|Kavernous Angiomen|Gehiraneurysmen | März 2008 | Phase 1 |
| NCT03337932 | Universitéit Medical Center Ljubljana | Erythema Chronicum Migrans | Januar 1, 2018 | Net applicabel |
| NCT00568711 | Dong-Min Kim|Chosun Universitéit Spidol | Scrub Typhus | September 2006 | Net applicabel |
| NCT01874860 | Universitéit vu Louisville | James Graham Brown Cancer Center | Colorectal Kriibs|Kapp- an Hals Kriibs | August 2013 | Phase 2 |
| NCT01171859 | IRCCS Policlinico S. Matteo | Transthyretin Amyloidose | Juli 2010 | Phase 2 |
| NCT01653522 | D'Cleveland Klinik | Migräne Stéierungen|Kappwéi, Migrän|Migrän|Migränesch Kappwéi|Migrän mat Aura|Migrän ouni Aura|Kappwéi Stéierungen, Primär | Juli 2012 | Net applicabel |
| NCT01820910 | International Extranodal Lymphoma Study Group (IELSG) | Marginal Zone Lymphoma vun Ocular Adnexal | Mäerz 2013 | Phase 2 |
| NCT01323101 | Universitéit vu Südkalifornien | Cystesch Fibrose | Abrëll 2008 | Phase 4 |
| NCT00829764 | Teva Pharmaceuticals USA | Gesond | Oktober 2006 | Phase 1 |
| NCT01668498 | AIO-Studien-gGmbH | Ras-Wildtype Colorectal Cancer | Mee 2011 | Phase 2 |
| NCT01030666 | Peter Eickholz|Heidelberg University|Dr. August Wolff GmbH & Co. KG Arzneimittel|Gaba International AG|Goethe University | Parodontitis | Abrëll 2007 | Phase 4 |
| NCT00012688 | US Department of Veterans Affairs|Colgate-Periogard-Dentsply|VA Office of Research and Development | Diabetis mellitus | Schlecht glycemesch Kontroll | Peridontal Krankheet | Net applicabel | |
| NCT01885910 | Derm Research, PLLC|WFH MEDICAL, LLC | Akne Vulgaris | Juli 2013 | Phase 4 |
| NCT02328469 | University Medical Center Ljubljana|Slowenian Research Agency|University of Ljubljana School of Medicine, Slowenien|Harvard University | Aseptesch Meningitis | Juni 2014 |
|
| NCT00355602 | Universitéit Dundee|Tenovus Schottland | Colitis, Ulcerativ | Juli 2006 | Net applicabel |
| NCT02606032 | Hamilton Health Sciences Corporation|Hamilton Academic Health Sciences Organisation | Ulcerativ Kolitis | Mee 2016 | Phase 2 |
| NCT01465802 | Pfizer | Non Small Cell Lung Cancer (NSCLC) | 26. Dezember 2011 | Phase 2 |
| NCT02623959 | MD Anderson Cancer Center | Fortgeschratt Kriibs|Malignant Pleural Effusiounen | 27. Abrëll 2016 | Phase 4 |
| NCT03481972 | IRCCS Policlinico S. Matteo | TTR Häerz Amyloidose | Abrëll 11, 2018 | Phase 3 |
| NCT00428818 | Universitéit vun Texas Southwestern Medical Center | Infektioun | August 2005 | Net applicabel |
| NCT01935622 | Virginia Commonwealth Universitéit | Non-ischemesch Kardiomyopathie | Systolesch Häerzversoen (NYHA II-III) | Juli 2012 | Phase 2 |
| NCT01886560 | Sun Yat-sen Universitéit | Eye Burns | September 2013 | Phase 2|Phase 3 |
| NCT04239755 | Damanhour Universitéit | Tanta Universitéit | Traumatesch Gehir Verletzung | Dezember 15, 2019 | Phase 4 |
| NCT02204254 | Centre Hospitalier Universitaire de Nice | Rosacea | Mäerz 2014 | Net applicabel |
| NCT00837213 | Stiefel, a GSK Company|GlaxoSmithKline | Akne | August 2007 | Phase 4 |
| NCT03115177 | Rush Universitéit Medical Center | Osteoarthritis | November 2015 | Net applicabel |
| NCT03618108 | Cadrock Pty. Ltd.|Center for Digestive Diseases, Australien | Coronar Häerzkrankheeten | Chlamydophila Pneumoniae Infektiounen | Abrëll 4, 2018 | Phase 2 |
| NCT03435952 | MD Anderson Cancer Center|BioMed Valley Discoveries, Inc|Merck Sharp & Dohme Corp. | Malignant Neoplasma vun der Brust|Malignant Neoplasms vun Verdauungsorganer|Malignant Neoplasme vum Auge Gehir an aner Deeler vum Zentralnervensystem|Malignant Neoplasme vu weibleche Genitalorganer|Malignant Neoplasme vu schlecht definéierten Sekundären an Onspezifizéierten Siten Siten|Malignant Neoplasme vu Lip Oral Kavitéit a Pharynx|Malignant Neoplasme vu männleche Genitalorganer|Malignant Neoplasme vu Mesothelial a Soft Tissue|Malignant Neoplasme vun Atmungs- an Intrathoracesch Organer|Malignant Neoplasme vun der Schilddrüs an aneren endokrinen Tränen|Malignant Neoplasme vun Urinarynären | 10. Juli 2018 | Phase 1 |
| NCT01867294 | Academic and Community Cancer Research United|National Cancer Institute (NCI) | Fortgeschratt Malignant Neoplasma|Dermatologesch Komplikatioun | 31. August 2012 | Phase 2 |
| NCT01677286 | Boston Universitéit | Amyloidose | Juli 2012 | Phase 2 |
| NCT00511875 | Thomas Gardner|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetesch Retinopathie | Juli 2008 | Phase 2 |
| NCT04108897 | Johns Hopkins Universitéit | Rosacea | September 17, 2019 | Fréier Phase 1 |
| NCT00631501 | Kaunas University of Medicine|University Hospital, Linkoeping | Lateral Epicondylalgie (Tennis Ielebou) | Net applicabel | |
| NCT02203682 | Sun Yat-sen Universitéit | Graves Ophthalmopathie|Graves Krankheet|Aen Krankheeten|Schilddrüsekrankheeten|Endokrine System Krankheeten|Aen Krankheeten, Hereditär|Hyperthyroidismus|Autoimmun Krankheeten|Immunsystem Krankheeten | Juli 2014 | Phase 2 |
| NCT02005653 | Indian Council of Medical Research | Filarial; Infestatioun | Februar 2009 | Phase 4 |
| NCT03585140 | Centro Dermatológico Dr Ladislao de la Pascua | Akne Vulgaris | Diätmodifikatioun | 1. Januar 2016 | Net applicabel |
| NCT02147262 | University Medical Center Ljubljana|University of Ljubljana School of Medicine, Slowenien|Medical University of Vienna|Harvard University | Chronesch atrophesch Acrodermatitis | Juli 2013 | Net applicabel |
| NCT02220751 | Universitéit vu Sao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontitis | Typ 2 Diabetis mellitus | März 2009 | Phase 3 |
| NCT01825408 | Universitéit vu North Carolina, Chapel Hill | Sinusitis | Februar 2013 | Phase 4 |
| NCT02884713 | King Faisal Spezialist Spidol & Fuerschung Center | GASTRITIS | Juni 2013 | Net applicabel |
| NCT02726646 | Universitéit vu Campinas, Brasilien|Pontificia Universidade Catolica de Sao Paulo | Chronesch Parodontitis | Juni 2015 | Phase 2 |
| NCT00883818 | Samsung Medical Center | Iwweraktiv Blase | Januar 2007 | Phase 4 |
| NCT00829790 | Teva Pharmaceuticals USA | Gesond | Oktober 2006 | Phase 1 |
| NCT01949233 | Universitéit Oxford|Oxford University Hospitals NHS Trust | Marfan Syndrom | Oktober 2013 | Phase 2 |
| NCT01518192 | Universitéit Medical Center Ljubljana|Slowenesch Fuerschungsagentur | Erythema Migrans|Post-Lyme Krankheet Symptomer | Juni 2006 | Phase 4 |
| NCT02845024 | Islamesch Azad Universitéit, Teheran | Diabetis mellitus mat Periodontal Krankheet | September 2014 | Net applicabel |
| NCT01879930 | Universitätsklinikum Inselspital, Bern | Chronescht Pelvic Pain Syndrom|Bladder Pain Syndrom | November 2012 | Phase 4 |
| NCT00041977 | CollaGenex Pharmaceuticals | Akne Rosacea | Juni 2002 | Phase 3 |
| NCT02341209 | Rochester General Hospital | Kutan T-Zell Lymphom|Mycosis Fungoides|Sezary Syndrom | 6. Februar 2018 | Phase 2 |
| NCT00002872 | Eastern Cooperative Oncology Group|National Cancer Institute (NCI)|North Central Cancer Treatment Group | Metastatesch Kriibs | November 1996 | Phase 3 |
| NCT03162497 | Medical University of Vienna | Dréchent Auge Syndrom|Meibomian Gland Dysfunktioun | 8. Januar 2018 | Phase 4 |
| NCT01418742 | Gesellschaft fur Medizinische Innovation ? Hamatologie und Onkologie mbH|ClinAssess GmbH | Colorectal Karzinom | August 2011 | Phase 2 |
| NCT00980148 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Chlamydial Infektioun | Dezember 2009 | Phase 3 |
| NCT03342456 | Den Drëtte Xiangya Spidol vun der Central South University|Livzon Pharmaceutical Group Inc.|Yung Shin Pharm. Ind. Co., Ltd. | Duodenal Ulcus Wéinst Helicobacter Pylori | 13. Dezember 2017 | Phase 4 |
| NCT04310930 | D'Universitéit vu Queensland|Australian Government Department of Health|Children's Hospital Foundation|Cystic Fibrosis Foundation|Newcastle University|Griffith University|Erasmus Medical Center|Monash University|University of Copenhagen|Hôpital Cochin|South Australian Health and Medical Research Institute|University vu Melbourne|James Cook University, Queensland, Australien|Murdoch Childrens Research Institute | Pulmonalerkrankheet duerch Mykobakterien (Diagnos) | Mäerz 2020 | Phase 2|Phase 3 |
| NCT03709459 | Kirby Institut|South Australian Health and Medical Research Institute|Monash University | STIs Präventioun | Dezember 17, 2019 |
|
| NCT04067011 | Emergent BioSolutions|Biomedical Advanced Research and Development Authority | Anthrax | August 12, 2019 | Phase 2 |
| NCT02844634 | British Columbia Center fir Krankheet Kontroll | HIV|Syphilis | Mee 15, 2018 | Phase 4 |
| NCT00647959 | Mylan Pharmaceuticals | Gesond | März 2006 | Phase 1 |
| NCT00170222 | Medical Center Alkmaar | Chronesch obstruktiv Pulmonalerkrankheet | Juli 2002 | Phase 4 |
| NCT03075891 | Galderma | Rosacea | 5. Juli 2017 | Phase 4 |
| NCT00031499 | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Syphilis | Juni 2000 | Phase 3 |
| NCT01205464 | Linkoeping Universitéit | Middegkeet|Radikulär Schmerz|Kognitiv Dysfunktioun|Parästhesie|Paresis | Februar 2005 | Net applicabel |
| NCT01301586 | D'Geschicht vun Nexgen Dermatologics, Inc. | ACNE VULGARIS | November 2010 | Phase 1|Phase 2 |
| NCT02305940 | Imperial College London | Chronic Obstructive Pulmonary Disease (COPD) | Juli 2014 | Phase 3 |
| NCT00351182 | Dong-Min Kim|Chosun Universitéit Spidol | Scrub Typhus | September 2005 | Phase 3 |
| NCT03334682 | Nantes Universitéit Spidol | Akne Vulgaris | 31. Januar 2018 | Phase 3 |
| NCT01788215 | Universitéit vu Rochester | Polycystescht Ovarial Syndrom (PCOS)|Irreguläre menstruellen Zyklen|Androgen Iwwerschoss | November 2010 | Phase 3 |
| NCT03076281 | Sidney Kimmel Cancer Center an der Thomas Jefferson University|Thomas Jefferson University | Larynx|LIP|Oral Kavitéit|Pharynx | Abrëll 3, 2017 | Phase 2 |
| NCT00439166 | Hamilton Health Sciences Corporation|The Physicians' Services Incorporated Foundation|McMaster University | Alzheimer Krankheet | Februar 2007 | Phase 3 |
| NCT02463942 | University Medical Center Ljubljana|University of Ljubljana School of Medicine, Slowenien | Tick-borne Encephalitis | September 2014 | Net applicabel |
| NCT00803842 | Northwestern Universitéit | Net kleng Zell Lung Cancer | Oktober 2008 | Net applicabel |
| NCT02086591 | Universitéit vu Rochester | Erwuessener Diffuse Grouss B-Zell Lymphom|Mantelzell-Lymphom Widderhuelend|Lymphom, Follikulär|Marginal Zone B-Zell-Lymphom|Malignant Lymphom - Lymphoplasmazytesch|Waldenstrom Macroglobulinemie|Kleng Lymphozytesch Lymphom|Chronesch Lymphozytesch Leukämie (CLL-Celllymphom)| | Mäerz 2014 | Phase 2 |
| NCT03980223 | Universitéit vu Kalifornien, San Francisco|Universitéit vu Washington|National Institute of Allergy and Infectious Diseases (NIAID)|Mayne Pharma International Pty Ltd|San Francisco Department of Public Health | Gonorrhea|Chlamydia|Syphilis | November 26, 2019 | Phase 4 |
| NCT00355459 | Universitéit vun Texas Southwestern Medical Center | Dréchent Auge Syndrom | August 2005 | Net applicabel |
| NCT01254799 | Omar Mamdouh Shaaban|Assiut Universitéit | Gebärmutterblutung | Januar 2008 | Phase 3 |
| NCT01547325 | NanoSHIFT LLC|Verdeedegungsministère vun den USA | Dehisced chirurgesch Wounds | Mee 2012 | Net applicabel |
| NCT00653380 | Par Pharmaceutical, Inc.|Anapharm | Fir d'Bioequivalenz ënner Fasting Bedéngungen ze bestëmmen | September 1999 | Phase 1 |
| NCT00635609 | Warner Chilcott | Akne Vulgaris | März 2008 | Phase 4 |
| NCT03765931 | Institut de Recherche pour le Developpement | Féiwer | Juli 2016 | Phase 4 |
| NCT01160640 | Harold Wiesenfeld|National Institute of Allergy and Infectious Diseases (NIAID)|University of Pittsburgh | Pelvic inflammatoresch Krankheet | November 2010 | Phase 2 |
| NCT01756833 | Universitéit vu Maryland, Baltimore | National Institut fir Alterung (NIA) | Aneurysmus | Mee 2013 | Phase 2 |
| NCT00688064 | Galderma | Schwéier Akne Vulgaris | August 2008 | Phase 3 |
| NCT01320033 | Galderma | Akne Vulgaris | 29. Mäerz 2011 | Phase 2 |
| NCT03397004 | St. Michael's Hospital, Toronto|Barrow Neurological Institute|Duke University|Feinstein Institut fir Medizinesch Fuerschung|Universitéit vu Pittsburgh|Sunnybrook Health Sciences Center | Hereditär hemorrhagesch Telangiektasie (HHT) | September 12, 2018 | Phase 2 |
| NCT01635530 | Turku Universitéit Spidol | Lyme Neuroborreliosis | August 2012 | Phase 4 |
| NCT03727620 | Mohammed V Souissi Universitéit | Aggressiv Parodontitis | 6. Januar 2014 | Phase 1|Phase 2 |
| NCT02688738 | Rothman Institut Orthopädie | Propionibacterium | Mäerz 2015 | Net applicabel |
| NCT00358462 | Universitéit vu Washington | National Institut fir Allergie an Infektiiv Krankheeten (NIAID) | Urethritis | Januar 2007 | Phase 3 |
| NCT02864550 | British Columbia Center fir Krankheet Kontroll | Syphilis|Sexuell iwwerdroen Infektiounen | August 15, 2019 | Phase 4 |
| NCT01595594 | Universitéit vu Sao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontal Krankheet | Typ 2 Diabetis | Mäerz 2010 | Phase 3 |
| NCT00964834 | PharmAthene, Inc.|National Institutes of Health (NIH)|Medarex|Quintiles, Inc.|Department of Health and Human Services | Anthrax | Juli 2009 | Phase 1 |
| NCT01809444 | Sun Yat-sen Universitéit | Thyroid Associéiert Opthalmopathie | November 2012 | Phase 2|Phase 3 |
| NCT01590082 | MD Anderson Cancer Center|National Institutes of Health (NIH)|National Cancer Institute (NCI) | Melanom | November 2012 | Phase 1|Phase 2 |
| NCT00207584 | Zentren fir Krankheet Kontroll a Präventioun | Mycoplasma Pneumoniae | Januar 1994 | Net applicabel |
| NCT00775177 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Gesond | Juni 2005 | Net applicabel |
| NCT03462329 | Universitéit Medical Center Ljubljana | Erythema Migrans | 1. Juni 2018 | Net applicabel |
| NCT00000403 | Indiana University|National Institut fir Arthritis a Muskuloskeletal a Hautkrankheeten (NIAMS)|National Institut fir Alterung (NIA) | Osteoarthritis | September 1996 | Phase 3 |
| NCT03508232 | Universitéit Alberta|Royal Alexandra Spidol | ST Segment Héicht Myokardinfarkt|Häerzfehler | Januar 6, 2020 | Phase 2 |
| NCT02553473 | Sorlandet Spidol HF | Neuroborreliosis, Borrelia Burgdorferi | Oktober 2015 | Phase 3 |
| NCT02207556 | Medical College vu Wisconsin | Primär systemesch Amyloidose | 1. Oktober 2014 | Phase 2 |
| NCT01783106 | Royal Liverpool University Hospital|National Association for Colitis and Crohn's Disease|National Institute for Health Research, Vereenegt Kinnekräich | Crohns Krankheet | 1. Februar 2014 | Phase 2 |
| NCT00353743 | Hospital de Clinicas de Porto Alegre | Ofdreiwung, Septik | Mee 2006 | Net applicabel |
| NCT01727973 | Sun Yat-sen Universitéit | Graves Ophthalmopathie|Graves Krankheet|Aen Krankheeten|Schilddrüsekrankheeten|Endokrine System Krankheeten|Aen Krankheeten, Hereditär|Hyperthyroidismus|Autoimmun Krankheeten|Immunsystem Krankheeten | Oktober 2012 | Phase 1|Phase 2 |
| NCT00857038 | Medical Center Alkmaar|Leiden University Medical Center|University of Amsterdam | Chronesch Obstruktiv Pulmonalerkrankheet | Entzündung | Lungenemphysem | Abrëll 2009 | Phase 4 |
| NCT02774993 | National University Hospital, Singapore|Tan Tock Seng Hospital|National University, Singapore|A*Star | Tuberkulos | September 2015 | Phase 2 |
| NCT03474458 | IRCCS Policlinico S. Matteo | Häerz AL Amyloidosis | 11. Februar 2019 | Phase 2|Phase 3 |
| NCT02874430 | Sidney Kimmel Cancer Center an der Thomas Jefferson University|Thomas Jefferson University | Brustkarzinom|Endometrial Clear Cell Adenocarcinoma|Endometrial Serous Adenocarcinoma|Uterine Corpus Cancer|Uterine Corpus Carcinosarcoma | 8. Juni 2016 | Phase 2 |
| NCT00016835 | Universitéit vu Michigan | National Institut fir Dental a Craniofacial Fuerschung (NIDCR) | Periodontal Krankheet|Diabetes Mellitus, Typ 2 | 17. Oktober 2001 | Phase 2 |
| NCT00064766 | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Endometrial Blutungen|Periodontal Krankheet | Februar 2003 | Phase 4 |
| NCT00803452 | Universitéit vu Louisville | Blepharitis | Juli 2008 | Phase 4 |
| NCT01434173 | Bayer|RTI Health Solutions | Drogen-induzéiert Liewer Verletzung | Juli 2001 |
|
| NCT00126204 | Barnes-Jewescht Spidol | Aorta Aneurysmus | März 2004 | Net applicabel |
| NCT01917721 | Hawaii Pazifik Gesondheet | Kawasaki Krankheet | Koronar Aneurysmus | Oktober 2013 | Phase 2 |
| NCT02775695 | Medical College vu Wisconsin | Resectable Bauchspaicheldrüs Kriibs | Abrëll 3, 2017 | Phase 2 |
| NCT03824340 | Aljazeera Spidol | Onfruchtbarkeet | Januar 30, 2019 | Net applicabel |
| NCT01847976 | Ottawa Hospital Research Institute|Canadian Breast Cancer Foundation | Péng | August 2013 | Phase 2 |
| NCT02850913 | Makerere Universitéit|Universitéit vun Oxford | Krampfadern | September 5, 2016 | Phase 2 |
| NCT00764361 | NanoSHIFT LLC | Diabetis Féiss Ulcer | Januar 2009 | Phase 2 |
| NCT02036528 | D'Geschicht vun Royer Biomedical, Inc. | Diabetesch Fouss Geschwëster | Januar 2014 | Phase 1|Phase 2 |
| NCT01661985 | Ostergotland County Council, Sweden|Statens Serum Institut | Urethritis|Cervicitis|Genital Mycoplasma Infektioun|Chlamydia Trachomatis | Februar 2010 | Phase 4 |
| NCT01380483 | Par Pharmaceutical, Inc.|Anapharm | Fir d'Bioequivalenz ënner Fasting Bedéngungen ze bestëmmen | Januar 2000 | Phase 1 |
| NCT00648180 | Mylan Pharmaceuticals | Gesond | Juli 2005 | Phase 1 |
| NCT01426269 | Galderma Laboratories, LP | Rosacea | September 2011 | Phase 4 |
| NCT02753426 | Universitéit vu Kalifornien, San Francisco | Chronesch Nier Krankheet | Kardiorenal Syndrom | Abrëll 2016 | Phase 1 |
| NCT02583282 | Postgraduate Institut fir Medizinesch Ausbildung a Fuerschung | Malignant Pleural Effusioun | 1. August 2015 | Net applicabel |
| NCT02927496 | D'Task Force fir Global Gesondheet | United States Agency for International Development (USAID) | Lymphödem | Lymphatesch Filariasis | Filariasis | 19. Juni 2018 | Phase 3 |
| NCT00652795 | Par Pharmaceutical, Inc.|Anapharm | Fir d'Bioequivalenz ënner Fasting Bedéngungen ze bestëmmen | Juli 2004 | Phase 1 |
| NCT03956212 | University Medical Center Ljubljana|University of Ljubljana School of Medicine, Slowenien | Erythema Migrans | 1. Juni 2017 | Net applicabel |
| NCT00855595 | Bayer | Papulopustulär Rosacea | Februar 2009 | Phase 4 |
| NCT03457636 | Derm Fuerschung, PLLC | Akne | 19. Mäerz 2018 | Phase 4 |
| NCT02894268 | Sir Run Run Shaw Spidol | Helicobacter Pylori Infektioun | Februar 2016 | Phase 4 |
| NCT03465774 | MD Anderson Cancer Center | National Cancer Institute (NCI) | Malignant Pleural Effusioun | 8. Mäerz 2018 | Fréier Phase 1 |
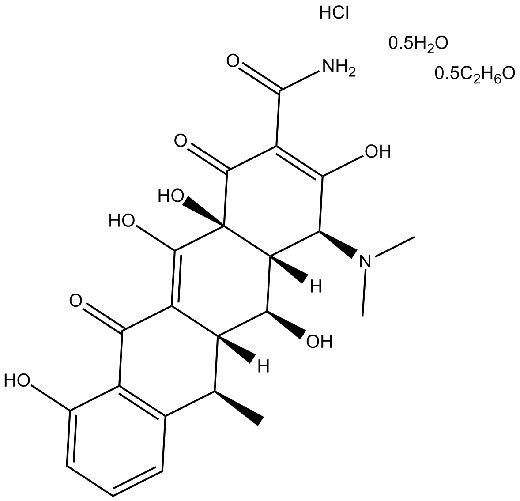
Chemesch Struktur





Propositioun18Qualitéit Konsistenz Evaluatioun Projeten déi guttgeheescht hunn4,an an6Projete sinn ënner Genehmegung.

Fortgeschratt international Qualitéitsmanagement System huet zolidd Fëllement fir Verkaf geluecht.

Qualitéitsiwwerwaachung leeft duerch de ganze Liewenszyklus vum Produkt fir d'Qualitéit an den therapeuteschen Effekt ze garantéieren.

Professional Regulatory Affairs Team ënnerstëtzt d'Qualitéitsfuerderunge wärend der Uwendung an der Umeldung.


Korea Countec Fläsche Verpackungslinn


Taiwan CVC Flasche Verpackungslinn


Italien CAM Board Packaging Line

Däitsch Fette Compacting Machine

Japan Viswill Tablet Detektor

DCS Kontrollraum







